

Racial and Ethnic Diversity in FDA Regulated Clinical Trials
Artificial intelligence has been in the spotlight recently, but it is not a new concept to the Food and Drug Administration (FDA) or the medtech industry. AI advancements in the…

Millions of patients’ lives are improved every day because of advancements in medical technology. Medical technology companies strive to innovate and develop the best tools to diagnose and treat patients. Discover the true impact of our industry.
59%
Cut heart disease fatalities by 59%
1/2
Days spent in hospitals cut in half
4000+
4,000+ diagnostic tests available today
2M
2 million U.S. jobs

Hot Topic
AdvaMed is collaborating with leaders and medtech champions from both parties in both houses of the 118th Congress on our top medtech priorities to foster greater medical innovation and expand access to medical technologies.
Latest Research
Our experts are continually conducting new and valuable research for the medtech industry.



Artificial intelligence has been in the spotlight recently, but it is not a new concept to the Food and Drug Administration (FDA) or the medtech industry. AI advancements in the…


Artificial intelligence has been in the spotlight recently, but it is not a new concept to the Food and Drug Administration (FDA) or the medtech industry. AI advancements in the…
Per- and polyfluoroalkyl substances, known as PFAS, are a broad class of over 12,000 substances that are found in a variety of consumer, commercial and industrial products, including medical devices…

The final report of a three-part series investigates the prevalence of racial and ethnic disparities in the use of in the use of advanced interventions in the Medicare program.

Kevin Lobo, Chairman and CEO, Stryker“As an AdvaMed member, Stryker has benefited tremendously from the access we have to so many resources.”

Tracy MacNeal, President & CEO, Materna Medical“We’ve had access to issues and networks in regulatory, health economics and fiscal policy that we would never have had as an independent medical device company.”

Martha Shadan, President & CEO, Miach Orthopaedics“The opportunities to engage, learn and stay informed through membership in AdvaMed is invaluable to us as a start-up organization.”
AdvaMedDx is the world leader in advocating for health care policies that support diagnostic testing through robust innovation, timely regulatory approval, appropriate payer coverage, payment rates commensurate with impact on care, and rapid adoption of new, safe and effective diagnostic tests.


Dedicated to the needs of smaller medical technology companies, AdvaMed Accel works to ensure the voice of small pioneering and entrepreneurial companies is heard by policymakers.
AdvaMed Digital Health Tech promotes the critical role of data and digital medical technologies in improving patient care. The Center provides digital medical technology companies opportunities to explore sector trends, challenges, and opportunities; network with and learn from peers; and shape public policy positions to advance digital medical technology contributions to health care.

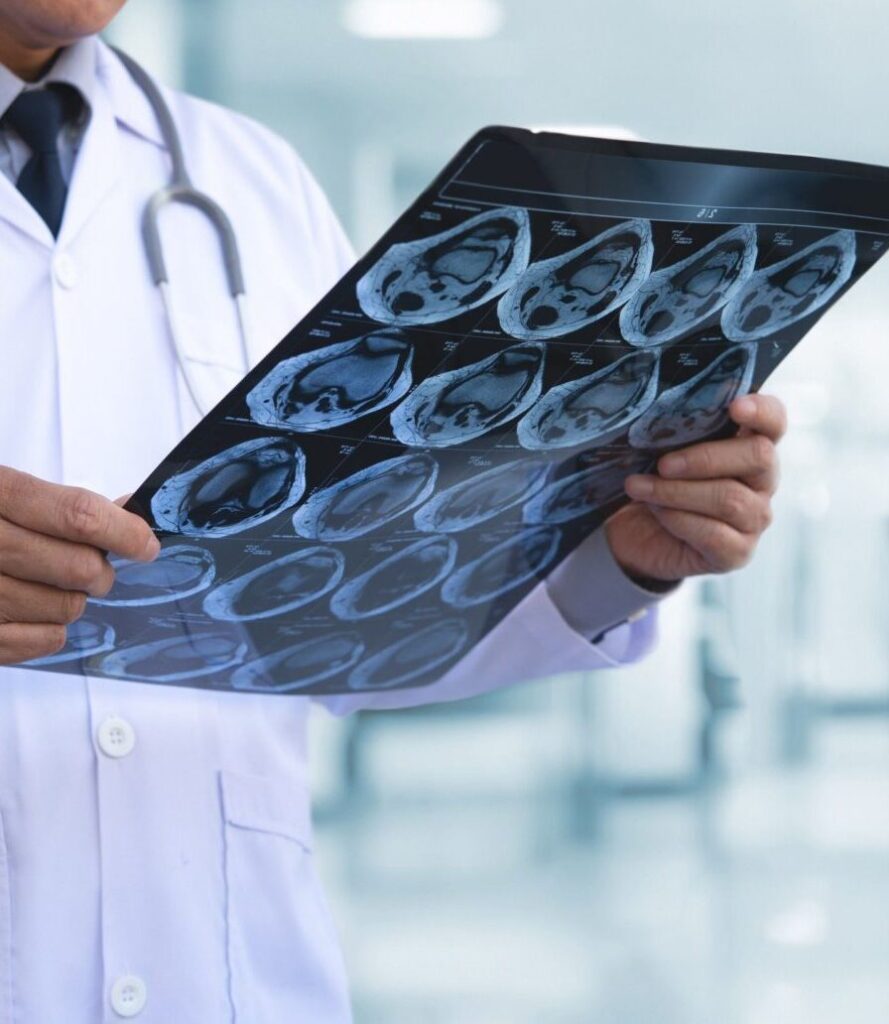
AdvaMed’s newest division — Medical Imaging – represents the world’s largest, leading companies that are providing healthcare professionals with the ability to screen, diagnose, treat, and monitor patients by providing accurate, detailed images.
MedtechVets helps veterans find meaningful employment in the medical technology industry. Sponsored by AdvaMed, this nonprofit bridges the gap for veterans by providing personalized career transition services, mentorship, career development guidance, and industry networking.

Join over 400 medical technology companies as a member of one of the most effective trade associations in Washington, D.C.